Are you struggling with properly dissolving hydroxypropyl methylcellulose (HPMC)1 in your formulations? The solubility characteristics of this versatile polymer can be tricky to navigate, leading to lumpy mixtures, inconsistent viscosity, and production delays.
HPMC is a water-soluble cellulose ether2 that dissolves readily in cold water but forms gels in hot water. It can also dissolve in certain polar organic solvents like methanol and ethanol when properly dispersed, making it versatile for various applications from construction to pharmaceuticals.

Understanding HPMC's unique solubility behavior is crucial for achieving optimal performance in your applications. Let's explore the four key aspects of HPMC solubility that will help you achieve better results in your formulations.
How Does HPMC Dissolve in Water?
Ever noticed how HPMC clumps when you add it directly to water? This happens because the outer particles immediately hydrate and form a gel barrier, preventing water from reaching the inner particles.
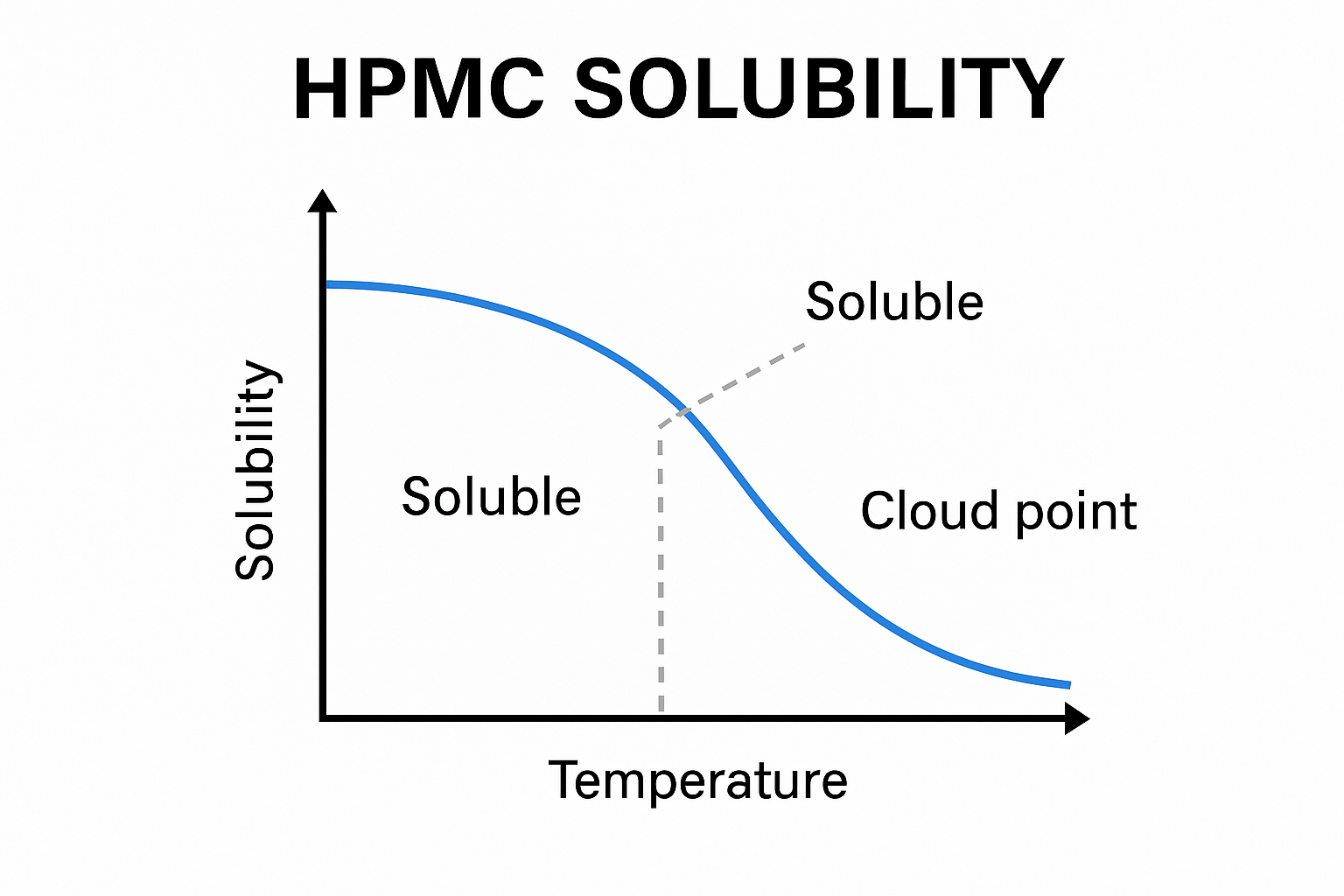
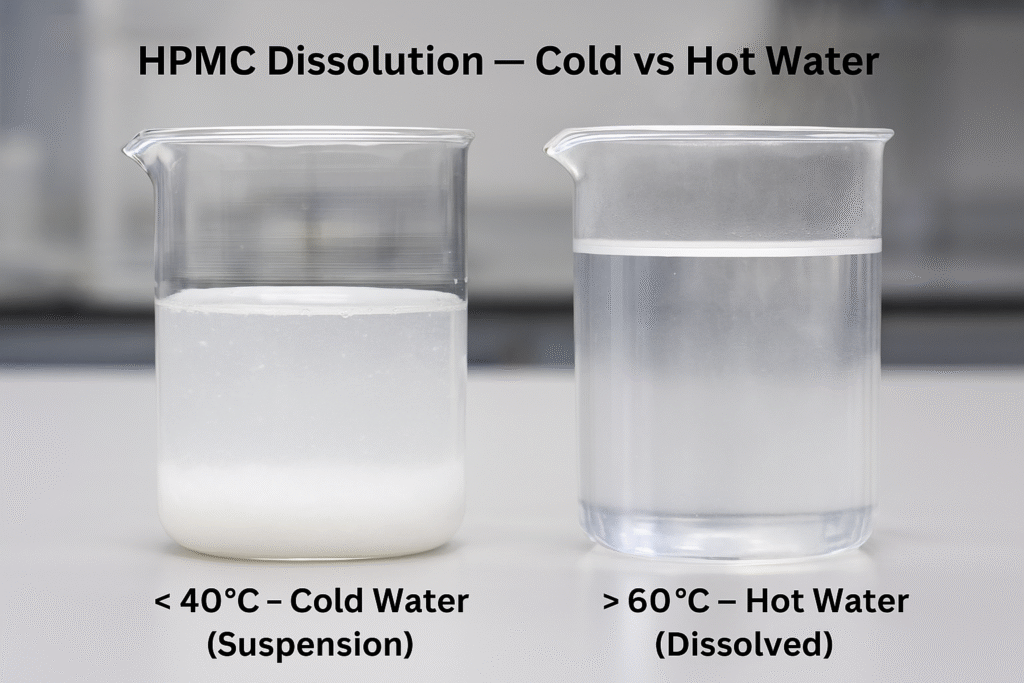
HPMC exhibits inverse solubility behavior - it dissolves well in cold water (below 40°C) but becomes insoluble at higher temperatures (above 60°C). When HPMC is added to hot water, it initially disperses without forming lumps, then dissolves as the water cools, creating a smooth solution.

This unique temperature-dependent solubility is what makes HPMC so valuable in various applications. The polymer's hydroxypropyl and methyl groups interact differently with water at different temperatures, allowing for controlled gelation. When dispersed in hot water, HPMC particles remain separate until cooling occurs, at which point they hydrate evenly and create a uniform solution.
I've found that understanding this temperature relationship is critical when working with HPMC. During one project, we were experiencing inconsistent viscosity in our formulation until we realized our water temperature was fluctuating. By strictly controlling the temperature during the dissolution process, we achieved remarkably consistent results.
The dissolution process also depends on the HPMC grade. Higher viscosity grades (with higher molecular weights) generally take longer to dissolve completely than lower viscosity grades. For optimal dispersion, the water should be either very cold (below 10°C) or hot (above 70°C), avoiding the intermediate temperature range where partial gelation can occur.
Dissolving HPMC: Methods and Considerations
Trying to find the most efficient way to dissolve HPMC? The right method depends on your specific application and equipment availability.
There are three effective methods for dissolving HPMC: the hot water method (where HPMC is dispersed in hot water and then cooled), the dry powder mixing method3 (blending HPMC with other dry ingredients before adding water), and the organic solvent wetting method (using solvents like ethanol for initial dispersion before adding water).

The hot water method is particularly effective for achieving lump-free solutions. The process typically involves heating water to approximately 70°C, slowly adding HPMC while stirring, and then allowing the mixture to cool while continuing to stir. This approach prevents the formation of the gel barrier that typically causes lumping with direct cold water addition.
The dry powder mixing method offers practical advantages for large-scale industrial applications. By blending HPMC with other dry ingredients before adding water, the HPMC particles become separated from each other, preventing them from forming lumps when water is added. The recommended ratio is typically 3:1 or 7:1 (other dry powders to HPMC).
For specialized applications, the organic solvent wetting method can be beneficial. This involves first dispersing HPMC in a compatible organic solvent like ethanol or isopropyl alcohol, then adding water to complete the dissolution process. This technique is particularly useful when formulating products that already contain organic solvents or when extremely rapid dissolution is required.
I once worked with a construction material manufacturer who was struggling with inconsistent mortar performance. By switching from direct cold water addition to the dry powder mixing method, they were able to achieve more reliable HPMC dispersion and significantly improve product consistency. The key was ensuring thorough dry blending before water addition, which prevented the formation of undispersed HPMC chunks in their final product.
Why Is HPMC Solubility in Cold Water Different from Hot Water?
Have you ever wondered why HPMC behaves so differently in cold versus hot water? This unusual property is key to many of its applications.
In cold water (below 40°C), HPMC gradually hydrates and dissolves to form a clear, viscous solution. In contrast, at higher temperatures (above 60°C), HPMC becomes insoluble and forms a thermal gel. This temperature-dependent solubility is due to the weakening of hydrogen bonds between water molecules and HPMC as temperature increases.
Two types of HPMC are available based on their cold water solubility characteristics: surface-treated and non-surface-treated. Surface-treated HPMC (often called "instant" grade) contains crosslinking agents like glyoxal that allow it to disperse quickly in cold water without immediate viscosity development. After approximately two minutes of stirring, the solution thickens as the HPMC fully hydrates.
Non-surface-treated HPMC4 behaves differently in cold water. When added directly to cold water, the outer layer of particles immediately forms a gel, encapsulating the inner particles and significantly slowing down complete dissolution. This leads to the formation of "fish-eyes" or lumps that can be difficult to dissolve completely.
Temperature plays a critical role in the hydration mechanism of HPMC. At low temperatures, water molecules form stronger hydrogen bonds with the hydroxyl groups in HPMC, facilitating solubilization. As temperature increases, these hydrogen bonds weaken, and hydrophobic interactions between HPMC molecules become dominant, leading to precipitation and gel formation.
When working with a pharmaceutical formulation, I discovered that pre-blending non-surface-treated HPMC5C](https://xhhpmc.com/what-makes-hpmc-hydroxypropyl-methyl-cellulose-essential-for-modern-construction/)[^4] with sugar helped prevent gel formation during dissolution by separating the HPMC particles. This simple technique reduced our production time significantly while maintaining the desired viscosity in the final product.
How Does HPMC Dissolve in Organic Solvents?
Working with organic solvents and need to incorporate HPMC? Understanding its solubility in different organic media is essential for successful formulation.
HPMC can dissolve in polar organic solvents like methanol, ethanol, and isopropyl alcohol, but is generally insoluble in pure (anhydrous) forms of these solvents. It exhibits good solubility in mixed water-organic solvent systems where water content is sufficient (typically 30-40%), making it useful for formulations like hand sanitizer gels.
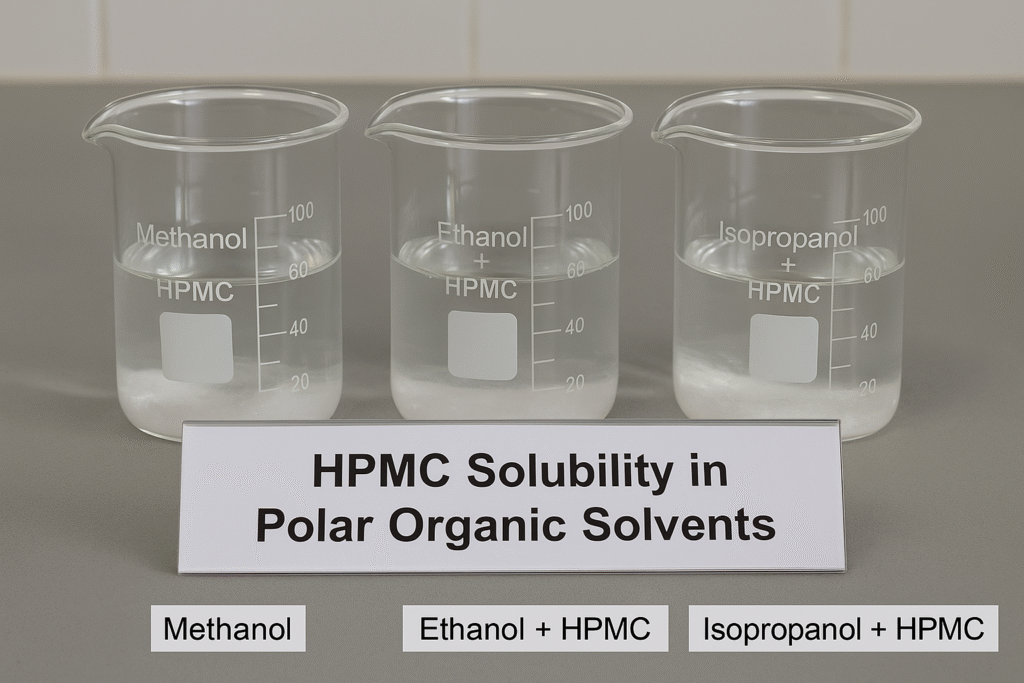
The solubility of HPMC in organic solvents depends on several factors including the molecular weight of the polymer, the degree of substitution of hydroxypropyl and methyl groups, and the polarity and hydrogen-bonding properties of the solvent. Generally, HPMC with lower molecular weight and higher hydroxypropyl substitution shows better solubility in organic media.
For practical applications involving organic solvents, a common approach is to first disperse HPMC in the organic solvent (like ethanol or isopropanol) and then add cold water while stirring continuously. This technique is particularly effective for formulations like hand sanitizer gels, where HPMC serves as a thickening agent in a high-alcohol environment.
HPMC is soluble in specific organic solvent systems:
- Methanol: Moderate solubility, better with some water content
- Ethanol: Soluble in aqueous ethanol but insoluble in anhydrous ethanol
- Isopropyl alcohol (IPA): Soluble when water is present
- Acetone: Limited solubility, better in mixed systems with water
- Chlorinated solvents: Generally poor solubility
I recently developed a formula for an alcohol-based hand sanitizer where we needed to achieve proper thickening without compromising the alcohol content. By first dispersing HPMC in the isopropyl alcohol and then adding the exact calculated amount of cold water required for proper dissolution, we maintained the required 70% alcohol content while achieving excellent gel consistency. This approach prevented the lumping that occurred when we tried adding HPMC directly to the alcohol-water mixture.
Conclusion
Understanding HPMC's solubility behavior in water and organic solvents is crucial for optimizing its performance across diverse applications. By selecting the appropriate dissolution method and controlling temperature, you can successfully incorporate this versatile polymer into your formulations.
-
Explore the versatility of HPMC in various industries, from construction to pharmaceuticals. ↩
-
Learn about the unique characteristics of cellulose ethers and their applications. ↩
-
Learn about this practical method for large-scale applications and its benefits. ↩
-
Find out how surface treatment enhances the solubility of HPMC in cold water. ↩
-
Understand the challenges and solutions for working with non-surface-treated HPMC. ↩