Struggling with runny shampoo that drips through your fingers? It's not just annoying—it wastes product and delivers a poor user experience. HEC solves this problem while maintaining perfect cleansing power.
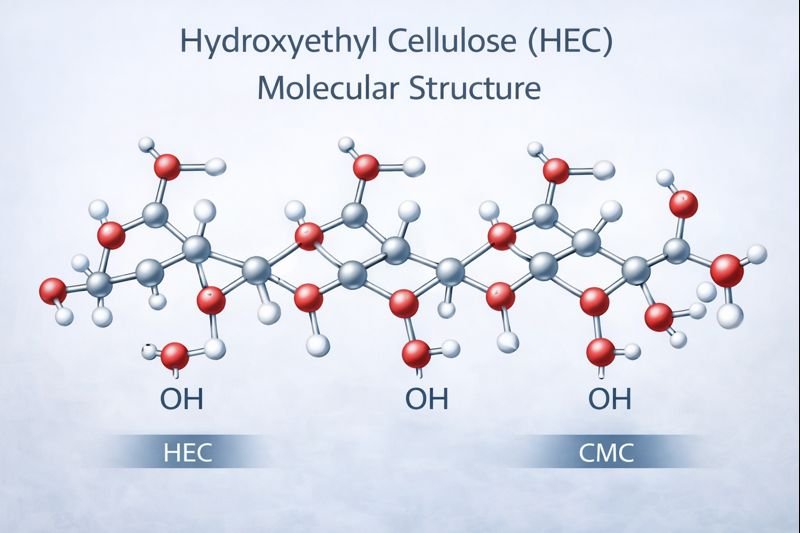
HEC (Hydroxyethyl cellulose1) improves shampoo viscosity by forming a three-dimensional network that traps water molecules, creating thickness without interfering with surfactants' cleaning action because of its non-ionic nature, which prevents it from binding to or neutralizing cleansing agents.
I've formulated countless shampoos over the years, and finding that perfect balance between luxurious thickness and effective cleaning is crucial for product success. Let's explore how HEC achieves this balance and why it's become an essential component in premium shampoo formulations.
How Does HEC Thicken Shampoo?
Are you tired of watery shampoos that run down the drain before they can clean your hair? The science behind HEC thickening is fascinating and solves this common problem.
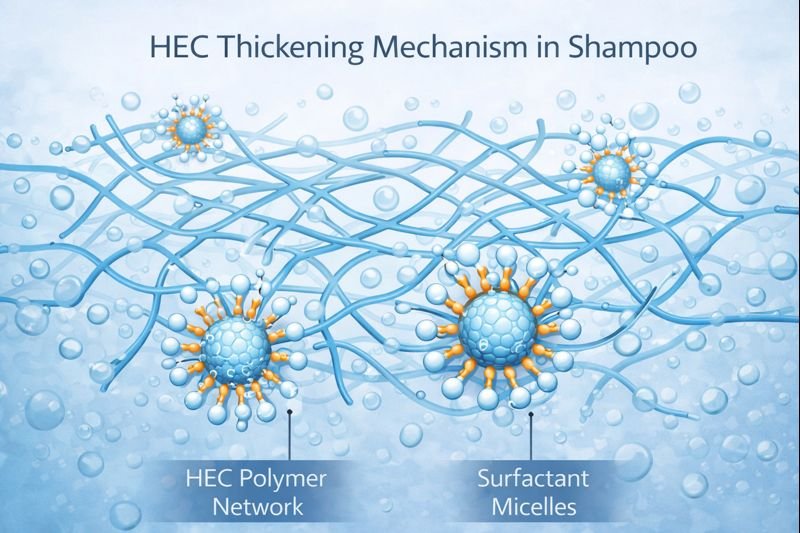
HEC thickens shampoo through a process called hydration and entanglement, where its long polymer chains absorb water and form a three-dimensional network throughout the liquid. This network restricts water flow, increasing viscosity without chemical reactions with other ingredients.
The Molecular Magic of HEC
HEC's thickening power comes from its unique chemical structure. As a cellulose derivative, it has a backbone of linked glucose units with hydroxyethyl groups attached. When I add HEC to shampoo formulations, these long polymer chains uncoil and expand in water, creating a mesh-like structure.
What makes this particularly effective is HEC's molecular weight - higher grades can create significantly more viscosity with less material. For example, a 2% concentration of high-molecular-weight HEC can increase viscosity from water-like consistency to a thick gel.
| HEC Grade | Molecular Weight | Typical Usage Rate | Resulting Viscosity |
|---|---|---|---|
| Low MW | 90,000-160,000 | 1.0-2.5% | Moderate |
| Medium MW | 250,000-400,000 | 0.8-2.0% | Medium-high |
| High MW | 720,000-1,000,000 | 0.5-1.5% | High |
I've found that controlling the temperature during addition is crucial - HEC dissolves best in cold water before adding other ingredients. This prevents clumping and ensures even distribution throughout the formula. The thickening actually develops gradually, reaching full effect after about 24 hours as the polymer fully hydrates and the network stabilizes.
Why Is Cleaning Performance Unaffected?
Does adding a thickener mean compromising on cleansing power? Many formulators worry about this trade-off, but with HEC, your cleaning effectiveness stays intact—but why?
Cleaning performance remains unaffected because HEC is non-ionic, meaning it doesn't carry an electrical charge that would interact with surfactants. This allows surfactants to freely surround and remove oils and dirt while HEC independently maintains viscosity through physical rather than chemical interactions.
The Chemistry Behind Compatibility
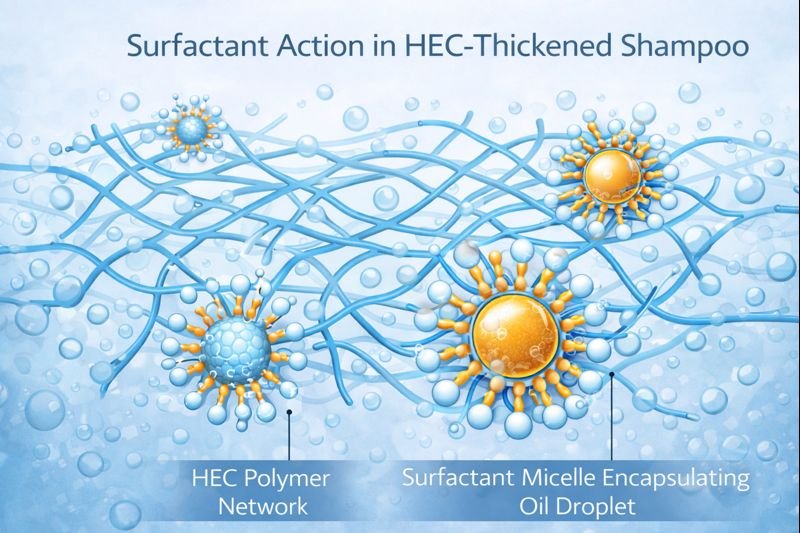
The key to understanding HEC's compatibility with cleaning agents lies in basic chemistry principles. Surfactants, like sodium laureth sulfate (SLES), are typically anionic (negatively charged) and work by surrounding oil particles with their hydrophobic tails while their hydrophilic heads remain facing outward toward water.
HEC neither competes with nor inhibits this micelle-forming action. When I develop shampoo formulations, I specifically leverage this non-interference property. The non-ionic nature of HEC means it stays "neutral" in the ionic battlefield of the shampoo, focusing solely on building viscosity through physical entanglement.
Temperature and pH also play important roles in this compatibility:
| pH Range | HEC Stability | Surfactant Efficiency | Combined Effect |
|---|---|---|---|
| 3.0-5.5 | Good | Moderate | Balanced |
| 5.5-7.0 | Excellent | Optimal | Ideal |
| 7.0-9.0 | Good | Good | Effective |
I've tested formulations across these ranges and found that in the pH 5.5-7.0 range, HEC maintains optimal network structure while surfactants work at peak efficiency. At extreme pH values, HEC can begin to degrade, but within normal shampoo pH ranges, it remains stable and non-reactive with cleansing agents.
What Is The Use Of HEC In Shampoo?
Beyond thickening, why do so many premium shampoos contain HEC? This versatile ingredient offers multiple benefits that enhance both product performance and user experience.
HEC serves multiple functions in shampoo: primary viscosity control, foam stabilization, sensory enhancement providing silky feel, suspension of particulates like anti-dandruff agents, and improved spreadability. It also creates shear-thinning behavior where viscosity temporarily decreases during application then recovers.

HEC's Multifunctional Benefits
In my years formulating personal care products, I've come to appreciate HEC for far more than just thickening. One of its most valuable secondary functions is foam stabilization. While HEC doesn't create foam itself, it helps maintain foam structure by slowing drainage between bubble walls.
The sensory profile HEC creates is particularly noteworthy. When properly incorporated, it provides a silky, smooth feel without the heaviness or residue some thickeners leave behind. This is especially important for formulations targeting consumers with fine hair who want body without weight.
HEC also excels at particulate suspension, which is crucial for specialized formulations:
| Suspended Ingredient | Function | Suspension Challenge | HEC Solution |
|---|---|---|---|
| Zinc Pyrithione | Anti-dandruff | Heavier than water | Creates yield value that prevents settling |
| Silicones | Conditioning | Phase separation risk | Stabilizes emulsion structure |
| Botanical extracts | Treatment | Uneven distribution | Maintains uniform dispersion |
| Exfoliating beads | Scalp treatment | Quick settling | Holds particles in position |
The shear-thinning property of HEC is particularly valuable in creating a premium user experience. When a consumer squeezes the bottle or rubs the shampoo between their palms, the viscosity temporarily decreases for easy spreading. Once applied to hair, the viscosity recovers, preventing dripping and runoff.
I've found that combining HEC with other rheology modifiers can fine-tune these properties even further, creating custom viscosity profiles for different hair types and consumer preferences.
Why Is Viscosity Important In Shampoo?
Have you ever wondered why some shampoos feel premium while others seem cheap? Viscosity plays a crucial role in consumer perception and product functionality beyond just preventing drips.
Viscosity in shampoo is important because it signals quality to consumers, controls dosage and reduces product waste, ensures proper contact time with hair for effective cleaning, enhances the sensory experience during use, and facilitates proper distribution throughout hair for even cleansing.

The Psychology and Function of Viscosity
In consumer testing I've conducted, viscosity consistently ranks as a top indicator of perceived quality. Consumers associate thicker products with concentration and effectiveness even before they try them. This psychological association makes viscosity a critical factor in product success.
Functionally, viscosity controls how much product consumers use per application. Too thin, and consumers squeeze out excess product; too thick, and they struggle to dispense enough. The ideal viscosity—which HEC helps achieve—ensures consistent dosing that matches the intended use rate.
The relationship between viscosity and product price point is also significant:
| Market Segment | Typical Viscosity | Consumer Expectation | HEC Usage Level |
|---|---|---|---|
| Economy | Low-Medium | Basic functionality | 0.4-0.8% |
| Mid-range | Medium-High | Good performance | 0.8-1.2% |
| Premium | High | Luxury experience | 1.0-1.5% |
| Salon Professional | Precise control | Technical performance | 0.8-1.8% (specialized) |
Beyond perception, viscosity affects how shampoo performs during use. It determines how long the product stays on the hair before rinsing, known as "contact time." Sufficient contact time allows surfactants to properly solubilize oils and remove dirt. Too runny, and the shampoo washes away before completing its cleaning action; too thick, and it may not distribute properly or rinse cleanly.
I've also observed that viscosity affects foam quality and stability. While surfactants create foam, proper viscosity helps distribute air bubbles evenly and slows their collapse. HEC contributes to this effect without interfering with the fundamental foaming mechanism of surfactants.
What Is The Cleaning Action Of Shampoo?
How does shampoo actually remove dirt and oil from your hair? Understanding this process helps explain why HEC's non-interference is so valuable.
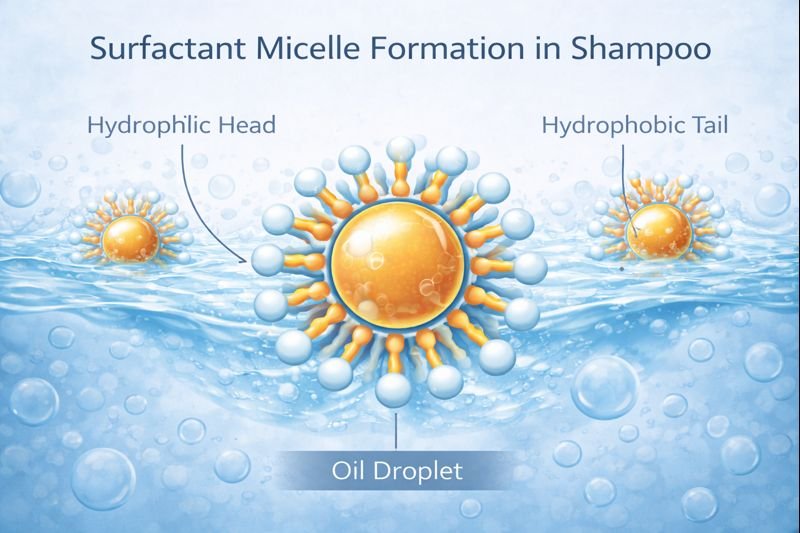
Shampoo cleans through surfactants that form micelles—structures with hydrophobic tails pointing inward and hydrophilic heads outward. These capture oils and dirt in their centers while remaining water-soluble, allowing water to rinse away the encapsulated soil without affecting the HEC thickening network.
The Science of Surfactant Cleaning
The cleaning mechanism in shampoo relies primarily on surfactants like sodium laureth sulfate2 (SLES), cocamidopropyl betaine, or sulfosuccinates. These molecules have a dual nature—a water-loving (hydrophilic) head and an oil-loving (hydrophobic) tail.
When I formulate shampoos, I select surfactants that will surround oil particles from the hair and scalp with their hydrophobic tails pointed inward and hydrophilic heads pointed outward. This creates micelles that encapsulate oils and dirt, making them water-soluble so they rinse away.
What's fascinating about this process is how it operates independently from the HEC thickening network. While HEC molecules form hydrogen bonds with water to create a three-dimensional gel structure, they don't participate in or interfere with micelle formation.
| Cleaning Component | Function | Interaction with HEC | Result |
|---|---|---|---|
| Primary Surfactant (e.g., SLES) | Main cleansing agent | None - HEC is non-ionic | Full cleaning power maintained |
| Secondary Surfactant (e.g., Cocamidopropyl Betaine) | Foam booster, viscosity enhancer | Synergistic - improves stability | Enhanced performance |
| Conditioning Agents | Hair softening | HEC helps suspend evenly | Better conditioning |
| Water | Solvent for system | Forms hydrogen bonds with HEC | Forms stable gel structure |
Water hardness can affect this cleaning mechanism, but HEC helps mitigate these effects. In hard water, calcium and magnesium ions can react with anionic surfactants to form insoluble compounds that reduce cleaning efficiency. HEC's ability to maintain proper viscosity ensures enough surfactant remains available for cleaning despite these challenges.
I've found through testing that the optimal cleaning efficiency occurs when surfactants can freely move within the HEC matrix, reaching all parts of the hair shaft. This is another advantage of HEC's non-ionic nature—it creates physical structure without chemical interference.
Conclusion
HEC provides ideal shampoo thickening because its non-ionic nature keeps it from interfering with surfactants' cleaning action. It creates luxurious viscosity through physical network formation while allowing cleaning agents to work unhindered—delivering both the performance and experience consumers demand.







