Ever noticed your homemade liquid detergent separating into layers? It's frustrating when ingredients settle at the bottom, affecting cleaning power and shelf appeal. This common problem has a simple solution.
To prevent separation in liquid detergents1, add hydroxyethyl cellulose (HEC)2 as a stabilizer at 0.2-0.5% concentration. Pre-disperse HEC in glycol or surfactant before adding to water, mix thoroughly at room temperature, and adjust pH to 6-8 for optimal performance.

Making stable liquid detergents requires understanding the science behind ingredient interactions. Let's explore how HEC can solve your separation issues and create professional-quality products that stay uniform from first use to last.
How to keep homemade liquid laundry detergent from separating?
Have you watched your carefully crafted laundry detergent split into ugly layers within days? This common problem ruins product performance and wastes your ingredients and time.
Homemade liquid laundry detergent separates because heavier ingredients settle over time. To prevent this, incorporate 0.2-0.5% HEC as a thickener and stabilizer, pre-wet it with glycerin or propylene glycol before adding to your formula, and ensure thorough mixing at moderate speeds.

The "pre-wet, then dissolve" rule is absolutely critical when working with HEC. I learned this the hard way when I first started formulating detergents. Never add HEC powder directly into water - it immediately forms clumps or "fish eyes" that are nearly impossible to dissolve. The proper technique is to first disperse HEC powder in propylene glycol, glycerin, or a small amount of surfactant (at about a 1:2 ratio) to create a uniform slurry. Then add this mixture to your main batch while stirring. This ensures HEC disperses evenly throughout the solution, forming a stable thickening network.
Finding the perfect addition rate is equally important. Through countless formulation tests, I've found that 0.2-0.5% is the sweet spot for most liquid detergents. Too little HEC, and you won't have enough suspension power to keep fragrance capsules and enzymes from settling. Too much, and your detergent becomes gel-like, making it difficult to pour and reducing its low-temperature solubility, leading to poor user experience.
HEC's salt tolerance is its greatest advantage in detergent formulations. Laundry detergents contain high levels of electrolytes (SLES, washing aids, etc.) which can deactivate many thickeners like carbomer. As a non-ionic polymer, HEC remains stable in high salt environments, maintaining consistent viscosity throughout the product's shelf life. This unique property makes it irreplaceable in detergent formulations where stability is paramount.
How to make high quality liquid detergent?
Does your liquid detergent lack the professional feel and stability of store brands? Creating salon-quality products requires understanding key ingredients and their precise functions.
To make high-quality liquid detergent, balance 15-30% primary surfactants (like SLES) with 5-10% secondary surfactants (like CAPB), add 0.2-0.5% HEC for stability, incorporate functional additives like enzymes, and adjust pH to 6-8 for optimal performance and shelf stability.

Creating truly exceptional liquid detergents requires attention to detail at every stage of formulation. When developing industrial-grade products for my clients, I focus on establishing the right foundation first. This means selecting appropriate surfactant systems that balance cleaning power with mildness. Typically, I start with sodium laureth sulfate (SLES)3 as my primary surfactant at 15-30%, then add cocamidopropyl betaine (CAPB) at 5-10% to boost foam stability and reduce irritation potential.
The stabilizing system is where HEC truly shines. For commercial-grade products, precise viscosity control is critical for both consumer perception and functional performance. I've found that incorporating HEC through a carefully controlled dispersion process yields the most consistent results. This involves creating a premix where HEC is fully hydrated before adding to the main batch, ideally using moderate-speed mixers that minimize air entrapment while ensuring thorough dispersion.
Temperature management is another crucial factor often overlooked in home formulations. Professional-grade detergents are typically produced with careful temperature control - I keep the mixture between 25-35°C during HEC incorporation, which balances dissolution speed with viscosity development. Too hot, and you risk degrading heat-sensitive ingredients like enzymes; too cold, and dissolution becomes inefficient.
Key Quality Parameters for Professional Liquid Detergents
| Parameter | Target Range | Function | HEC Impact |
|---|---|---|---|
| Viscosity | 300-1000 cP | Pour consistency, perceived quality | Primary controller |
| pH | 6.0-8.0 | Cleaning efficiency, ingredient stability | Stable across pH range |
| Stability | No separation for 12+ months | Shelf life, performance consistency | Prevents settling |
| Clarity | Clear to slightly opaque | Visual appeal | Minimal impact when properly dispersed |
By focusing on these technical parameters and leveraging HEC's unique properties, you can create liquid detergents that match or exceed commercial quality standards while maintaining stability throughout their shelf life.
What are the uses of HEC chemical?
Wondering if that HEC you bought has other applications? This versatile polymer does far more than just thickening detergents, making it a valuable addition to your formulation toolkit.
HEC (hydroxyethyl cellulose) is used as a thickener, stabilizer, and film-former in detergents, cosmetics, paints, and construction materials. It creates smooth textures, prevents ingredient separation, forms protective films, and improves product stability across varying pH levels and temperatures.

In my 15+ years working with cellulose derivatives, I've seen HEC's remarkable versatility firsthand across multiple industries. Beyond its primary role in liquid detergents, HEC serves as an essential ingredient in personal care formulations, where it creates the perfect viscosity for shampoos and body washes while stabilizing fragrance and active ingredients. Its non-ionic nature makes it particularly valuable in formulas containing high levels of electrolytes or challenging pH ranges where other thickeners might fail.
In the construction sector, HEC functions as a critical water retention agent in cement-based products. When incorporated into tile adhesives or renders at concentrations of 0.2-0.6%, it prevents water from being absorbed too quickly into porous substrates, giving cement particles sufficient time to hydrate properly. This results in stronger bonds and reduced cracking in the finished application. The same water-binding properties make HEC valuable in paint formulations, where it prevents premature drying during application and improves the final film's durability.
Pharmaceutical applications represent another significant use case. HEC creates the ideal texture for topical medications like gels and creams, controlling drug release rates through its film-forming abilities. In tablet manufacturing, it serves as a binder that holds ingredients together during compression while facilitating controlled dissolution when consumed. This multifunctionality across industries stems from HEC's fundamental chemical structure - a cellulose backbone modified with hydroxyethyl groups that create the perfect balance of water solubility and thickening efficiency.
HEC Concentration Guidelines by Application
| Application | Typical Concentration | Primary Function | Secondary Benefits |
|---|---|---|---|
| Liquid Detergents | 0.2-0.5% | Suspension/stabilization | Improved pour properties |
| Shampoos/Body Wash | 0.5-1.5% | Viscosity control | Enhanced feel, stabilization |
| Wall Paints | 0.3-0.8% | Thickening | Anti-settling, improved application |
| Construction Adhesives | 0.2-0.6% | Water retention | Extended working time |
| Pharmaceutical Gels | 1.0-3.0% | Gel formation | Controlled release of actives |
Understanding these diverse applications helps formulators leverage HEC's full potential, often solving multiple formulation challenges with a single ingredient while maintaining stability across diverse environmental conditions.
Why does detergent break down cell membranes?
Ever wondered why detergents are so effective at removing stubborn grease and stains? The answer lies in detergents' ability to disrupt cell membranes and lipid structures through specific chemical interactions.
Detergents break down cell membranes because their amphiphilic molecules contain both hydrophilic (water-loving) and hydrophobic (water-repelling) parts. This dual nature allows them to penetrate lipid bilayers, disrupt the membrane structure by replacing lipid-lipid interactions with detergent-lipid interactions, causing membrane disintegration.
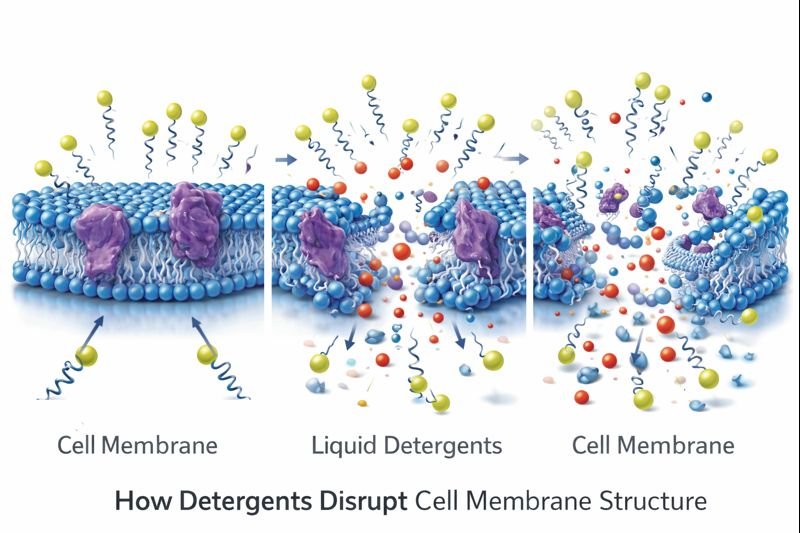
The science behind detergent action fascinates me as a formulation chemist. Cell membranes are primarily composed of phospholipids arranged in a bilayer structure - with hydrophilic heads facing outward toward aqueous environments and hydrophobic tails clustered inward. This arrangement creates a stable barrier essential for cellular function. Detergents exploit this architecture through their amphipathic structure, which mirrors phospholipids but with crucial differences in molecular geometry and solubility properties.
When detergents encounter cell membranes, the initial interaction occurs between the detergent's hydrophobic tails and the membrane's lipid interior. As concentration increases, detergent molecules progressively insert themselves between phospholipids, disrupting the tight packing and weakening the intermolecular forces that maintain membrane integrity. This process, known as intercalation, continues until reaching a critical concentration where the membrane structure becomes fundamentally compromised.
The severity of this disruption varies significantly depending on detergent type. Ionic detergents like sodium dodecyl sulfate (SDS) aggressively solubilize membranes through strong electrostatic and hydrophobic interactions, completely disintegrating cellular structures - explaining their use in lysing cells for protein extraction. Non-ionic detergents such as Triton X-100 interact more gently through primarily hydrophobic associations, often preserving protein tertiary structure while still disrupting membranes. Zwitterionic detergents like CHAPS represent a middle ground, offering moderate membrane disruption while maintaining protein functionality.
Detergent Types and Their Membrane Disruption Mechanisms
| Detergent Class | Examples | Disruption Mechanism | Typical Applications |
|---|---|---|---|
| Anionic | SLES, SDS | Strong ionic & hydrophobic interactions | Heavy-duty cleaning, complete cell lysis |
| Non-ionic | Triton X-100, Tween | Primarily hydrophobic interactions | Gentle cleaning, protein isolation |
| Zwitterionic | CHAPS, CHAPSO | Balanced hydrophobic/ionic interactions | Membrane protein extraction |
| Amphoteric | Cocamidopropyl betaine | pH-dependent interactions | Personal care products, mild cleaning |
Understanding these mechanisms helps explain why certain detergents excel at specific cleaning tasks while others might be better suited for different applications based on their membrane-disrupting properties and overall chemical behavior.
Conclusion
Using HEC correctly prevents separation in liquid detergents. Pre-wet it with glycol before adding to water, maintain 0.2-0.5% concentration, and leverage its salt tolerance for stable, professional-quality products that perform consistently throughout their shelf life.
FAQ
What concentration of HEC works best for liquid detergents1?
The optimal concentration range is 0.2-0.5%. This provides sufficient thickening and stabilization without creating overly viscous products.
Can I add HEC powder directly to my detergent mixture?
No, never add HEC powder directly. Always pre-disperse it in propylene glycol, glycerin or a surfactant at a 1:2 ratio before adding to the main batch.
How does HEC compare to other thickeners for detergents?
Unlike many thickeners, HEC maintains stability in high-electrolyte environments, making it ideal for detergent formulations containing surfactants and salts.
Will HEC affect the cleaning performance of my detergent?
When used at recommended levels, HEC stabilizes the formulation without interfering with cleaning performance or surfactant action.
What's the shelf life of detergents containing HEC?
Properly formulated detergents with HEC can maintain stability for 12-24 months, preventing ingredient separation throughout this period.







