Many of our customers struggle with properly dissolving hydroxypropyl methylcellulose (HPMC)1. Poor dissolution leads to lumps, inconsistent mixtures, and wasted material. Let me share what I've learned after years of manufacturing.
HPMC is a water-soluble cellulose ether2 that dissolves differently depending on temperature. Cold water causes it to hydrate and swell before dissolving, while hot water may cause lumping. The best dissolution typically involves dispersing HPMC in hot water (80-90°C) then cooling, or using special cold-water dispersible grades.

I've seen many formulators make simple mistakes when working with HPMC. Our factory has spent years perfecting the production of hydroxypropyl methylcellulose across our six manufacturing lines. Let's explore the key aspects of HPMC solubility that will make your production process smoother.
How Does HPMC Solubility in Water Work?
When I first started working with HPMC, I was confused by its unusual dissolution behavior. The powder would sometimes form frustrating lumps that took hours to dissolve.
HPMC exhibits inverse solubility in water - it dissolves readily in cold water but forms a gel in hot water (above its gel point, typically 65-85°C). This happens because at lower temperatures, water molecules can penetrate between the polymer chains, while at higher temperatures, hydrophobic interactions become dominant.
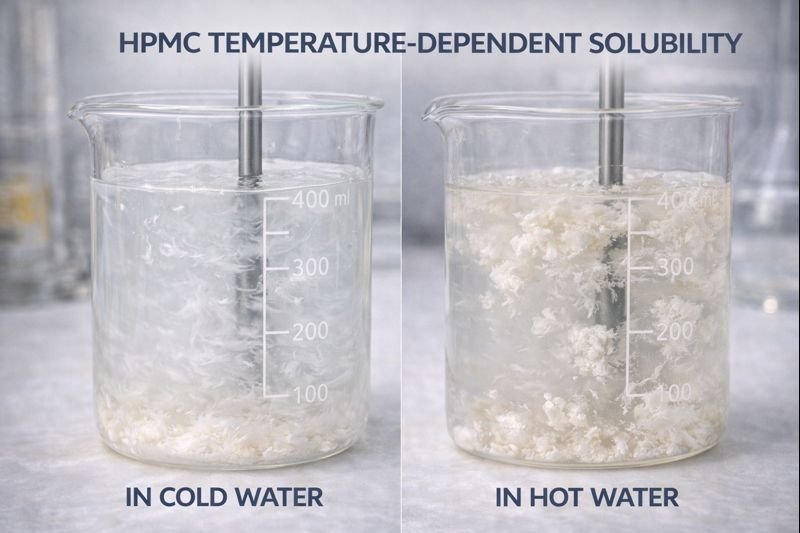
Looking deeper at HPMC's water solubility, we need to understand its unique molecular structure. The hydroxypropyl and methyl substitutions on the cellulose backbone create this temperature-dependent behavior3. During our manufacturing process at Kehao, we carefully control these substitution levels to achieve desired solubility profiles.
The viscosity grade also significantly affects dissolution rate. Lower viscosity grade4s (3-15 mPa·s) dissolve much faster than higher viscosity grades (100,000+ mPa·s). This is why careful selection of the right HPMC grade for your application is crucial.
Here's a breakdown of typical dissolution times by viscosity:
| HPMC Viscosity Grade | Approximate Dissolution Time (20°C) | Recommended Dissolution Method |
|---|---|---|
| Low (3-15 mPa·s) | 10-30 minutes | Direct addition to cold water |
| Medium (15-100 mPa·s) | 30-60 minutes | Hot/cold technique or high-shear mixing |
| High (>100 mPa·s) | 1-3 hours | Hot/cold technique with extended mixing |
From my experience supplying to mortar factories across Saudi Arabia and the UAE, I've found that proper dissolution significantly impacts final product quality and consistency.
What Are the Best Methods for Dissolving HPMC?
I once visited a customer in Pakistan who was frustrated with persistent lumps in their mortar mix. Their production was suffering, and they blamed our HPMC quality.
The best method for dissolving HPMC is the "hot/cold" technique: disperse HPMC powder in water heated above its gel point (about 80-90°C), which prevents immediate hydration. Then cool the mixture while stirring to allow gradual, uniform hydration. Alternatively, use specialized cold-water dispersible grades with surface treatment.
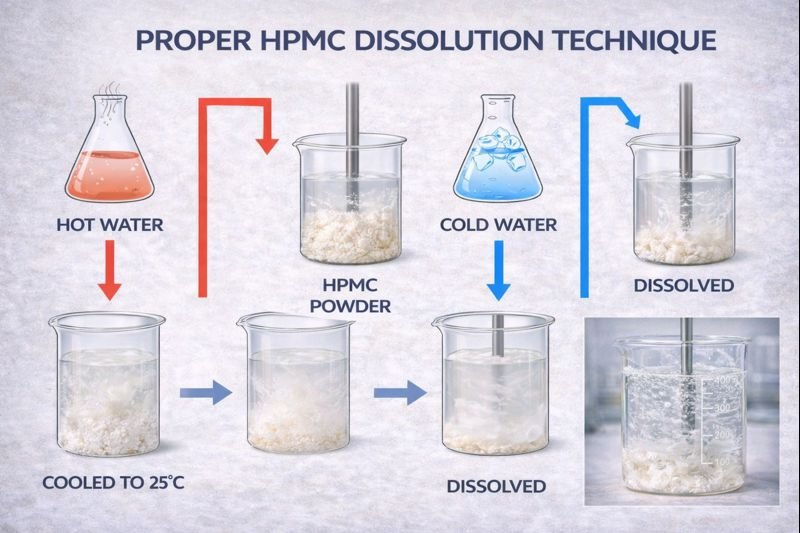
The dissolution method you choose depends on your specific manufacturing setup and requirements. At our Kehao production facility, we've helped hundreds of clients optimize their HPMC dissolution processes.
For industrial applications, several factors affect dissolution efficiency:
-
Agitation intensity: Higher shear forces break up particles more effectively. Mechanical stirrers, high-speed mixers, or homogenizers significantly reduce dissolution time.
-
Particle size: Our finer HPMC powders dissolve faster but are more prone to lumping if improperly added. Coarser grades disperse better but dissolve more slowly.
-
Water quality: Hard water with high mineral content can interfere with dissolution and reduce HPMC effectiveness.
-
Pre-blending with other dry ingredients: In dry-mix mortars, pre-blending HPMC with other powders (like cement or sand) before adding water helps prevent lumping.
When working with large batches in mortar production, I recommend creating a concentrated HPMC solution5 first (master batch), then diluting it into the final formulation. This approach has helped our customers in Brazil and Mexico achieve consistent results in their large-scale operations.
Why Is HPMC Solubility Different in Cold Water?
During a technical consultation with a client in India, I noticed they were wasting considerable time waiting for their HPMC to dissolve. They didn't understand how temperature affects dissolution.
In cold water (below 30°C), HPMC particles gradually hydrate from the outside in, allowing water to penetrate between polymer chains without forming lumps. The methoxyl groups become less hydrophobic at lower temperatures, enabling hydrogen bonding with water molecules and facilitating dissolution.

The cold-water dissolution mechanism of HPMC follows a specific pattern that depends on its chemical structure. When we manufacture HPMC at our Kehao facilities, we carefully balance the ratio of hydroxypropyl to methyl substitution, which directly affects cold water solubility.
HPMC with higher hydroxypropyl content6 generally shows improved cold water solubility compared to those with higher methoxyl content. This is because hydroxypropyl groups increase the hydrophilic character of the molecule, making it more compatible with water even at lower temperatures.
Temperature plays a critical role in dissolution kinetics:
| Water Temperature | Dissolution Behavior | Processing Considerations |
|---|---|---|
| 0-20°C | Slowest dissolution, no lumping | Requires longer mixing time, ideal for powdered products |
| 20-40°C | Moderate dissolution rate | Good balance of speed and processing ease |
| 40-60°C | Faster initially, risk of partial gelation | Careful monitoring required |
| >65°C | Gelation occurs, no true dissolution | Used for dispersion before cooling |
For our customers working in colder climates, like our partners in Georgia, we recommend slightly higher hydroxypropyl substitution levels in their HPMC to maintain good solubility even in cold water conditions. I've found that this small adjustment makes a significant difference in processing efficiency.
How Does HPMC Dissolve in Organic Solvents?
A paint manufacturer from Vietnam contacted me last year about using HPMC in their solvent-based systems. They were confused about its compatibility with non-aqueous solvents.
HPMC is generally insoluble in pure organic solvents but may dissolve in specific solvent mixtures. It's insoluble in hot water and most anhydrous alcohols but dissolves in certain binary organic solvent systems like dichloromethane/alcohol or water/alcohol mixtures, depending on the HPMC's substitution pattern.

When we examine HPMC solubility in organic solvents more closely, we find that its behavior depends on both the chemical nature of the solvent and the specific chemical structure of the HPMC. The methoxyl and hydroxypropyl substitution degrees determine how it interacts with different solvents.
HPMC solubility in common organic solvent systems:
| Solvent Type | Solubility | Applications |
|---|---|---|
| Acetone | Insoluble | Can be used for precipitation/purification |
| Methanol | Partially soluble (depends on DS) | Limited applications |
| Ethanol | Generally insoluble | Used in certain coating formulations |
| Water/Ethanol mixtures | Soluble (ratio-dependent) | Pharmaceutical coatings |
| Dichloromethane/Methanol | Soluble in specific ratios | Specialized film applications |
| Toluene | Insoluble | Not recommended |
For our customers in the pharmaceutical coating industry, we often supply HPMC grades specifically optimized for water/alcohol solvent systems. The balance of hydroxypropyl and methyl substitution is carefully controlled to achieve the desired solubility profile.
In paint applications, where organic solvents are common, HPMC is typically incorporated through an aqueous phase that is later combined with the solvent-based components. Our technical service team helps customers in Singapore and the Philippines develop these specialized formulation approaches.
Conclusion
Mastering HPMC solubility requires understanding its temperature-dependent behavior3, choosing appropriate dissolution methods, and selecting the right grade for your application. With these four key insights, you'll achieve better results in your formulations.
-
Explore this resource to understand HPMC's properties and its diverse applications in various industries. ↩
-
Learn about the advantages of water-soluble cellulose ethers and how they can enhance your formulations. ↩
-
This link will provide insights into how temperature influences solubility, crucial for HPMC handling. ↩ ↩
-
Explore how viscosity grades impact dissolution rates, which is vital for selecting the right HPMC. ↩
-
Learn about concentrated HPMC solutions and how they can streamline your production processes. ↩
-
Discover how hydroxypropyl content influences solubility, particularly in cold water applications. ↩







