Are you tired of construction materials that crack, building mortars that don't hold, or pharmaceutical coatings that dissolve too quickly? These problems stem from using the wrong binding agents. Cellulose ethers offer solutions to these challenges.
Cellulose ethers1 are modified natural polymers derived from cellulose through etherification processes. They function as thickeners, binders, film formers, and water retention agents in various industries. Their properties vary based on the type and degree of chemical substitution.
I've worked with cellulose ethers for over 15 years at our factory in China. What fascinates me most is how we transform a simple, abundant natural material - cellulose - into highly specialized industrial products through careful chemical modification. Let me take you through this remarkable transformation journey.
What Is the Chemical Structure of Cellulose Ethers?
When I first started manufacturing cellulose derivatives, I was confused by their complex molecular structure. How could such similar-looking white powders have such different properties in application?
Cellulose ethers1 are polymers created by replacing hydroxyl groups (-OH) in cellulose molecules with various ether groups. The backbone consists of β-D-glucose units linked by glycosidic bonds, with ether substituents determining specific properties.
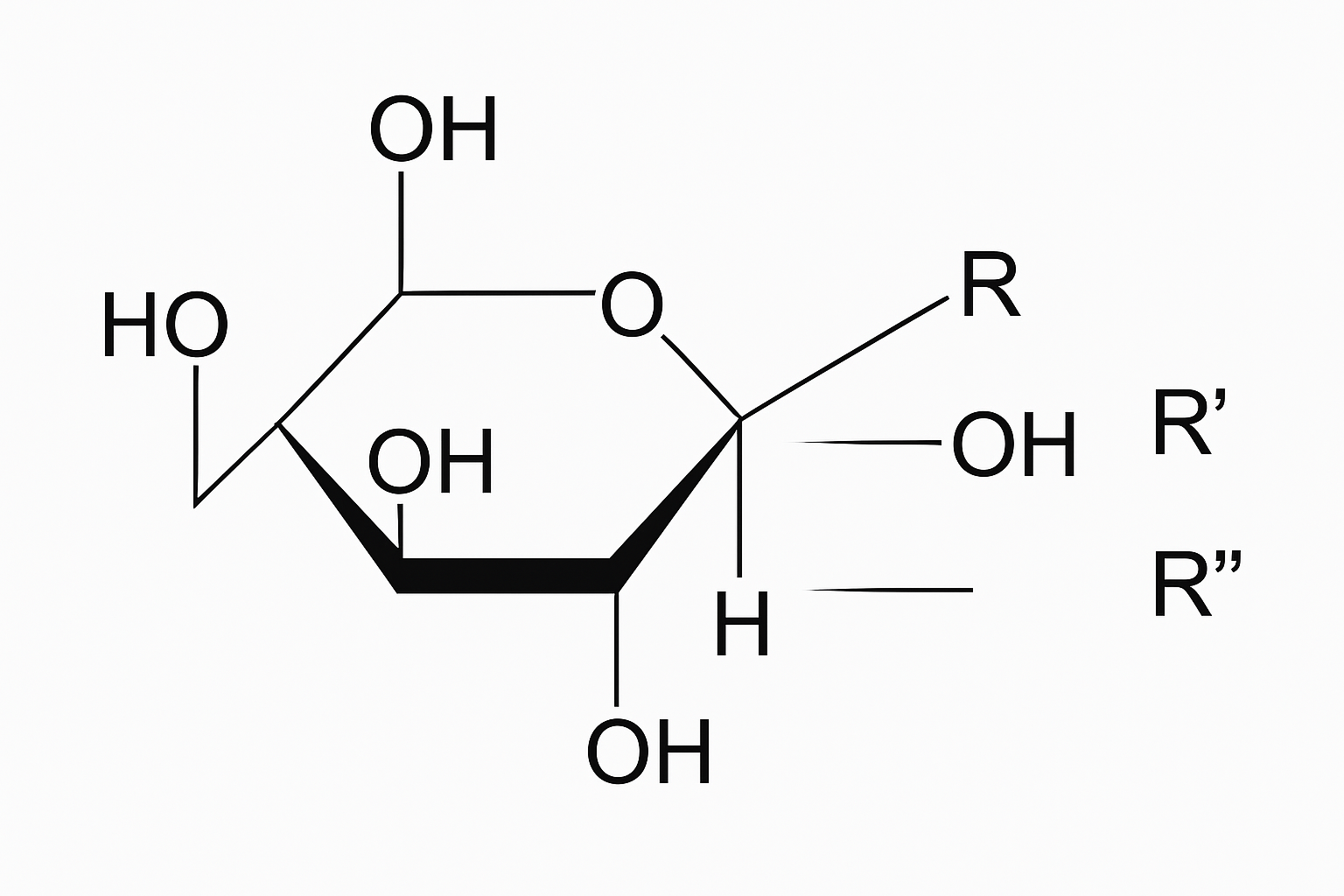
Understanding the Backbone and Substituents
Cellulose itself is nature's most abundant polymer, making up the cell walls of plants. Its backbone consists of linked glucose units forming long chains. Each glucose unit contains three hydroxyl (-OH) groups that can be modified through etherification.
The magic happens when we replace these hydroxyl groups with other functional groups through a process called etherification. Common substituents include:
- Methyl groups (-CH₃): Create methylcellulose (MC) and contribute to water solubility
- Hydroxypropyl groups (-CH₂CH(OH)CH₃): Add in hydroxypropyl methylcellulose (HPMC)2, improving solubility in cold water
- Carboxymethyl groups (-CH₂COOH): Found in carboxymethylcellulose (CMC)3, adding ionic character
- Hydroxyethyl groups (-CH₂CH₂OH): Present in hydroxyethylcellulose (HEC)4, enhancing water retention
Each substitution alters how the molecule interacts with water, changes its viscosity profile, and determines its compatibility with other materials. This is why we can make such a diverse range of products from the same base material.
What Are Degree of Substitution (DS)5 and Molar Substitution (MS)6?
In our factory, customers often ask why two batches of HPMC might perform differently despite having the same viscosity. The answer lies in the subtle details of substitution patterns.
Degree of Substitution (DS) indicates the average number of substituted hydroxyl groups per glucose unit (maximum 3.0). Molar Substitution (MS) represents the average number of moles of substituent added per glucose unit, potentially exceeding 3.0 for some groups.
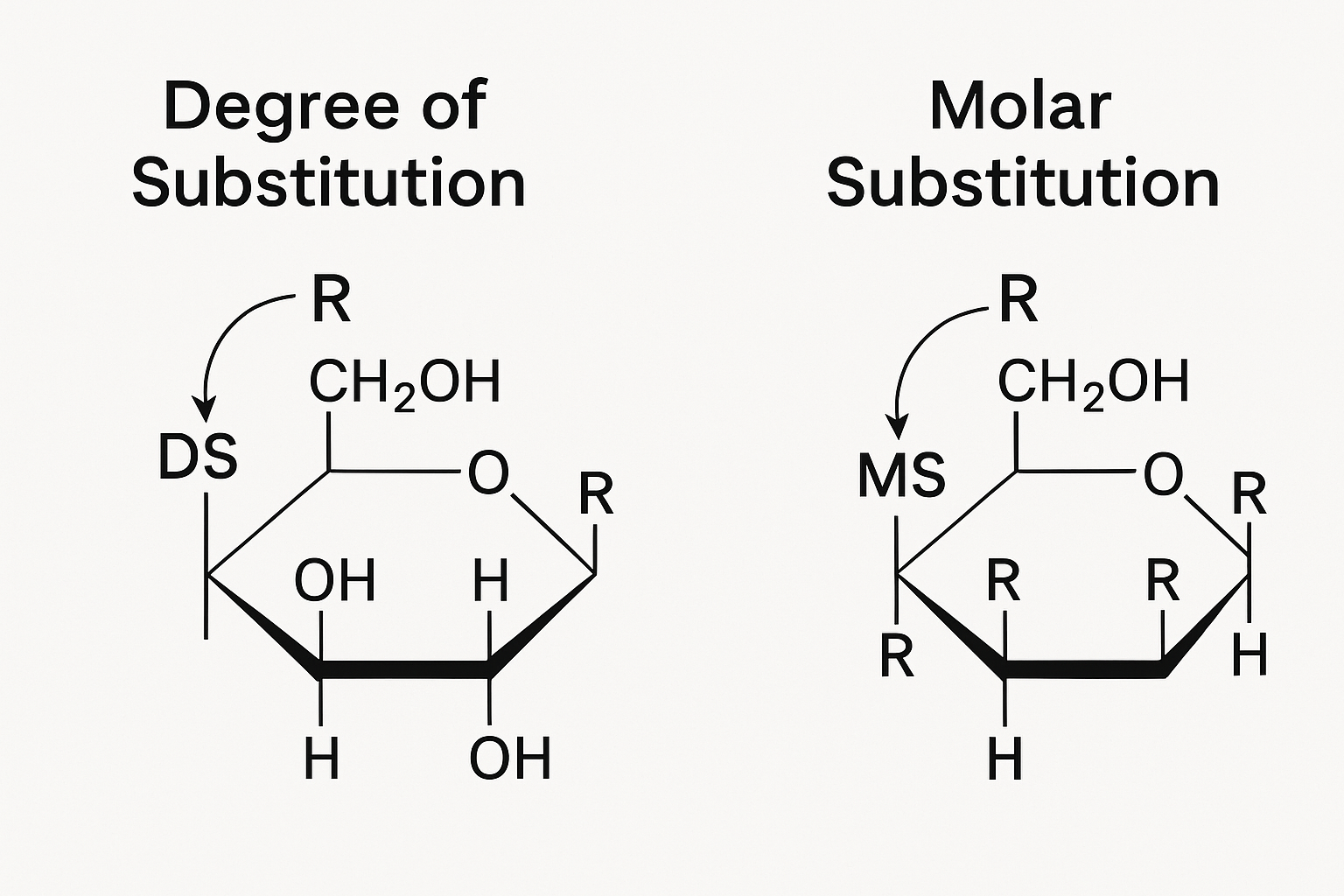
The Critical Difference Between DS and MS
Understanding the difference between DS and MS is essential for predicting how cellulose ethers will behave in applications. This distinction is especially important for hydroxypropyl and hydroxyethyl substitutions.
DS is limited to a maximum value of 3.0 because each glucose unit in cellulose has only three hydroxyl groups available for substitution. But with some substituent groups like hydroxypropyl, the added groups themselves contain hydroxyl groups that can undergo further substitution. This creates a cascade effect where MS can exceed 3.0.
| For example, in our HPMC production: | Parameter | Typical Range | Effect on Properties |
|---|---|---|---|
| DS (Methyl) | 1.4-2.0 | Controls water solubility temperature | |
| MS (Hydroxypropyl) | 0.1-1.0 | Affects surface activity and organic solubility |
The ratio of these parameters directly impacts performance characteristics. A higher methyl DS with lower hydroxypropyl MS creates products with higher thermal gelation temperatures - perfect for certain construction applications where delayed setting is beneficial.
What Are the Key Substituents and Their Functional Roles?
When I first consulted with a pharmaceutical company about tablet coatings, they were using the wrong type of cellulose ether. Their tablets dissolved too quickly, causing patient discomfort.
Different substituent groups create distinct cellulose ether types with unique properties. Methyl groups provide thermal gelation, hydroxypropyl adds cold water solubility, carboxymethyl enhances binding capacity, and hydroxyethyl improves flexibility and water retention.

How Substituents Transform Properties
Each substituent group we attach to cellulose creates dramatic changes in how the final product performs. This isn't just academic knowledge - it directly impacts how we customize products for specific applications.
Methyl (-CH₃) Groups:
These hydrophobic groups create interesting temperature-dependent solubility. Methylcellulose dissolves in cold water but forms gels when heated - a property we exploit in construction adhesives and food products. The methyl substitution disrupts hydrogen bonding at higher temperatures, causing polymer chains to associate and form a gel network.
Hydroxypropyl (-CH₂CH(OH)CH₃) Groups:
These larger groups prevent close chain association and improve solubility in both cold and hot water. In HPMC, they work alongside methyl groups to fine-tune gelation temperature, surface activity, and binding strength. Our construction clients particularly value hydroxypropyl substitution for its excellent water retention properties in cement-based mortars.
Carboxymethyl (-CH₂COOH) Groups:
These introduce ionic character, making CMC unique among cellulose ethers. The negative charges create electrostatic repulsion between chains, dramatically increasing water solubility and viscosity even at low concentrations. This makes CMC an excellent stabilizer in detergents, pharmaceuticals, and food products.
Hydroxyethyl (-CH₂CH₂OH) Groups:
These enhance hydrogen bonding with water molecules, giving HEC exceptional water retention capabilities. The film-forming properties make it valuable in personal care products and as a protective colloid in latex paints.
How Are Cellulose Ethers Produced Industrially?
The first time I walked through our production facility, I was amazed by the transformation of raw cotton linters into sophisticated chemical products. The process combines traditional chemical engineering with precise quality control.
Industrial production of cellulose ethers involves alkalizing cellulose with sodium hydroxide, reacting with etherifying agents under controlled conditions, neutralizing, purifying, drying, and milling. The process requires careful temperature, pressure, and reaction time management.

The Manufacturing Process Step-by-Step
Our factory operates six production lines that convert cellulose into different cellulose ether products. The process involves several critical stages, each carefully controlled to ensure consistent quality.
-
Raw Material Preparation: We start with high-purity cellulose, usually from cotton linters or wood pulp. The cellulose must be properly pretreated to remove lignin, hemicellulose, and other impurities.
-
Alkalization: The cellulose is treated with concentrated sodium hydroxide (18-50%) to form alkali cellulose. This critical step swells the cellulose fibers, making them more accessible for the subsequent etherification reaction.
-
Etherification: This is where the chemistry happens. Depending on the product we're making, we add different etherifying agents:
- Methyl chloride for MC and HPMC
- Propylene oxide for hydroxypropyl groups
- Monochloroacetic acid or its sodium salt for CMC
- Ethylene oxide for HEC
The reaction occurs in specialized reactors under precise temperature (50-120°C) and pressure conditions.
-
Neutralization and Purification: After etherification, the product contains unreacted reagents and by-products. We neutralize the mixture with acids, then wash it thoroughly to remove salts and other impurities. For pharmaceutical grades, this purification is especially rigorous.
-
Drying and Milling: The purified product is dried in specially designed dryers, then milled to achieve the desired particle size distribution. This affects dissolution rate and handling properties.
-
Quality Control: Every batch undergoes testing for:
- Viscosity (in various solutions)
- Substitution levels (DS and MS)
- Moisture content
- Particle size distribution
- Purity parameters
The entire process requires careful balancing of reaction conditions. For instance, increasing alkalization improves reactivity but can degrade cellulose chains if taken too far. This delicate balance is why expertise and experience are so crucial in cellulose ether manufacturing.
How Are Cellulose Ethers Used in Pharmaceuticals and Industry?
Our customers often tell me they're discovering new applications for our products. One client recently switched from synthetic polymers to our HPMC for tablet coatings, improving both production efficiency and patient satisfaction.
Cellulose ethers1 serve diverse functions across industries. In pharmaceuticals, they control drug release and form tablet coatings. In construction, they improve water retention and workability. In personal care, they thicken formulations, while in food, they stabilize and texturize products.
Diverse Applications Across Multiple Industries
The versatility of cellulose ethers comes from their ability to modify rheology, form films, retain water, and bind ingredients - all while being derived from sustainable sources. Here's how they function in different sectors:
Pharmaceutical Applications:
HPMC and MC excel in controlled-release drug formulations, where they form a gel matrix that regulates drug diffusion. The hydroxypropyl content can be adjusted to customize dissolution profiles. For enteric coatings, we recommend specific grades of HPMC that resist stomach acid but dissolve in intestinal fluid.
In tablet binding, low-viscosity grades help maintain tablet integrity without excessive hardness. Our pharmaceutical customers particularly value the consistent batch-to-batch performance we provide, as even small variations can affect drug release profiles.
Construction Materials:
In cement-based mortars and renders, HPMC and HEMC improve:
- Water retention (preventing rapid drying)
- Workability (making application easier)
- Adhesion to substrates
- Sag resistance (for vertical applications)
The hydroxypropyl content significantly impacts open time - critical for tile adhesives where installers need sufficient time to make adjustments.
Personal Care Products:
HEC and HPMC function as thickeners and stabilizers in shampoos, lotions, and creams. They create pleasant sensory properties without the stickiness of some synthetic polymers. Their film-forming capabilities also make them excellent for hair styling products.
Food Applications:
MC and HPMC serve as emulsifiers, stabilizers, and texturizers in various food products. Their thermal gelation properties are particularly valuable in fried foods, where they form a barrier that reduces oil absorption.
Who Are the Major Manufacturers and Global Suppliers of Cellulose Ethers?
When I attend international trade shows, customers often ask how our products compare to those from other global suppliers. Understanding the market landscape helps them make informed decisions.
The global cellulose ethers market includes major manufacturers like Dow, Ashland, Shin-Etsu, and medium-sized producers like our Kehao factory in China. Production capacity is concentrated in North America, Europe, and East Asia, with different regions specializing in particular grades.
The Global Supply Landscape
The cellulose ethers market has evolved significantly over the past two decades, with production capacity increasingly shifting toward Asia while specialized, high-performance grades remain concentrated in Western countries.
Major Global Players:
- Large Multinationals: Companies like Dow, Ashland, and Shin-Etsu dominate the high-end pharmaceutical and food-grade markets. They typically offer extensive technical support and regulatory documentation.
- Mid-sized Specialists: Our company, Kehao, falls into this category, along with several other Asian manufacturers. We focus on providing excellent quality-to-price ratio with customizable specifications.
- Regional Producers: Smaller companies serving local markets, often specializing in specific applications like construction.
| Regional Production Trends: | Region | Market Share | Specialization |
|---|---|---|---|
| East Asia | 45-50% | Construction grades, growing pharmaceutical capacity | |
| North America | 20-25% | Pharmaceutical and food grades, specialized CMC | |
| Europe | 15-20% | High-performance grades, regulated applications | |
| Others | 10-15% | Various applications |
The market is increasingly segmented by application rather than geography. For example, our factory exports to over 20 countries including Saudi Arabia, UAE, India, and Brazil, focusing primarily on construction and industrial grades.
Supply Chain Considerations:
Production capacity is only one aspect of the supply equation. Reliable logistics, consistent quality, technical support, and certification compliance are equally important factors. Our customers particularly value our ability to provide customized packaging and stable quality across production batches.
The recent global shipping challenges have highlighted the importance of supply chain resilience, prompting many buyers to develop relationships with multiple suppliers across different regions.
FAQs About Cellulose Ethers
Many potential customers reach me through Google searches, often with similar technical questions about cellulose ethers. I've compiled these common queries to help clarify key concepts.
Common questions about cellulose ethers involve biodegradability, safety profiles, viscosity measurement, substitution effects, and shelf life. These products are generally biodegradable, safe for human contact, available in various viscosity grades, customizable through substitution patterns, and stable for 2-3 years when properly stored.

Answering Your Most Common Questions
Are cellulose ethers biodegradable?
Yes, most cellulose ethers are biodegradable to varying degrees. Their biodegradability depends on the type and degree of substitution. Generally, lower substituted products biodegrade more readily. For example, CMC is more biodegradable than highly substituted HPMC. This makes them environmentally preferable to many synthetic polymers.
Are cellulose ethers safe for human contact?
Cellulose ethers have excellent safety profiles. Many grades are approved for food contact and pharmaceutical use by regulatory bodies like FDA and EFSA. They have low toxicity, minimal skin irritation potential, and aren't known to cause sensitization. This safety profile extends to environmental impact, where they pose minimal risk compared to synthetic alternatives.
How is the viscosity of cellulose ethers measured and specified?
Viscosity measurement is standardized but varies by cellulose ether type:
- For HPMC and MC: Measured as the viscosity of a 2% solution at 20°C using a rotational viscometer
- For CMC: Typically measured at 1% or 2% concentration
- For HEC: Usually measured at 1% concentration
The viscosity range for commercial products spans from 5 mPa·s to over 100,000 mPa·s, with each application requiring specific ranges. For example, tile adhesives typically use HPMC with viscosities between 15,000-75,000 mPa·s.
How do substitution patterns affect properties?
Beyond just DS and MS values, the distribution pattern of substituents along the cellulose backbone significantly affects performance. More uniform substitution typically improves solubility and solution clarity. The ratio between different substituent types (like methyl/hydroxypropyl ratio in HPMC) determines thermal gelation temperature, surface activity, and film flexibility.
What is the shelf life of cellulose ethers?
When properly stored in cool, dry conditions in sealed packaging, most cellulose ethers remain stable for 2-3 years. Factors that accelerate degradation include:
- Exposure to high humidity
- Elevated temperatures
- UV radiation
- Microbial contamination
For best results, we recommend storing products in their original packaging, away from direct sunlight, and in areas with relative humidity below 65%. Some customers in tropical climates opt for smaller package sizes to minimize exposure during use.
How do I select the right cellulose ether for my application?
Selection depends on several factors:
- Required functions (water retention, thickening, binding, film-forming)
- Processing conditions (temperature, pH, mixing equipment)
- Compatibility with other ingredients
- Regulatory requirements
- Cost constraints
For example, in exterior rendering mortars, we typically recommend HPMC with higher hydroxypropyl content for better water retention in hot, dry conditions. For interior jointing compounds, standard HPMC grades often provide sufficient performance at lower cost.
Can cellulose ethers be used in combination with other additives?
Yes, they work synergistically with many other additives. Common combinations include:
- With redispersible polymer powders in construction adhesives
- With carbomers in personal care for enhanced rheology
- With other gums in food applications for texture optimization
- With plasticizers in pharmaceutical coatings
In our laboratory, we routinely test compatibility to ensure optimal performance in customers' specific formulations.
Conclusion
Cellulose ethers represent a remarkable transformation of natural cellulose into versatile industrial ingredients. Through controlled chemical modification, we create products that thicken, bind, retain water, and form films across numerous applications. Their continued development promises even greater sustainability and performance benefits.
-
Explore the versatility of cellulose ethers in various industries and their unique properties. ↩ ↩ ↩
-
Explore the applications of HPMC in pharmaceuticals and construction. ↩
-
Learn about the unique properties and applications of CMC in various industries. ↩
-
Discover how HEC enhances water retention and film-forming properties. ↩
-
Understand how Degree of Substitution influences the properties of cellulose ethers. ↩
-
Find out how Molar Substitution affects the performance of cellulose derivatives. ↩







