Struggling with paint or coating products that lose thickness or form precipitates when mixed with hard water? This common problem costs manufacturers thousands in returns and complaints each year.
HEC (hydroxyethyl cellulose) thickener1 doesn't actually work "better" in hard water—it works consistently well in both hard and soft water because it's a non-ionic polymer. This chemical neutrality makes it unaffected by the calcium and magnesium ions that cause other thickeners to fail.

I've spent years helping customers solve formulation problems, and water hardness compatibility is among the most overlooked factors. Let me explain why HEC stands out in this regard, especially compared to other common thickening agents.
Why Does Hard Water Cause Poor Formation of Lather with Soap?
Washing your hands with soap in hard water often leaves that frustrating lack of lather and sticky residue. This happens because hard water contains minerals that sabotage the cleaning process.
Hard water contains calcium (Ca²⁺) and magnesium (Mg²⁺) ions that react with soap molecules, forming insoluble precipitates known as soap scum. These precipitates reduce lathering ability and leave deposits on surfaces, making cleaning less effective.

The chemistry behind this reaction is fascinating. Soap molecules are salts of fatty acids with a negatively charged head (carboxylate anion) and a hydrophobic tail. When soap meets hard water, the positively charged calcium and magnesium ions bind with the negatively charged soap molecules, creating those insoluble calcium and magnesium salts.
This is exactly why many customers notice regional variations in their product performance. I once worked with a paint manufacturer who couldn't understand why their formula worked perfectly in coastal regions but failed in areas with limestone bedrock. The culprit? Water hardness differences affecting their anionic thickener.
Common Problems with Different Water Hardness Levels
| Water Type | Hardness Range (ppm) | Effect on Anionic Thickeners | Effect on HEC |
|---|---|---|---|
| Soft | 0-60 | Works as intended | Works as intended |
| Moderately Hard | 61-120 | Slightly reduced effectiveness | No effect |
| Hard | 121-180 | Significantly compromised | No effect |
| Very Hard | 180+ | Mostly ineffective | No effect |
What Is the Difference Between HPMC and HEC?
Many customers ask me which cellulose derivative they should choose, often confused by technical jargon. Let's break down these important differences.
HPMC (hydroxypropyl methylcellulose)2 and HEC (hydroxyethyl cellulose) differ primarily in their functional groups. HPMC has both methyl and hydroxypropyl groups, while HEC has hydroxyethyl groups. This makes HPMC more resistant to enzymatic degradation and gives it better surface activity, while HEC offers better salt tolerance.
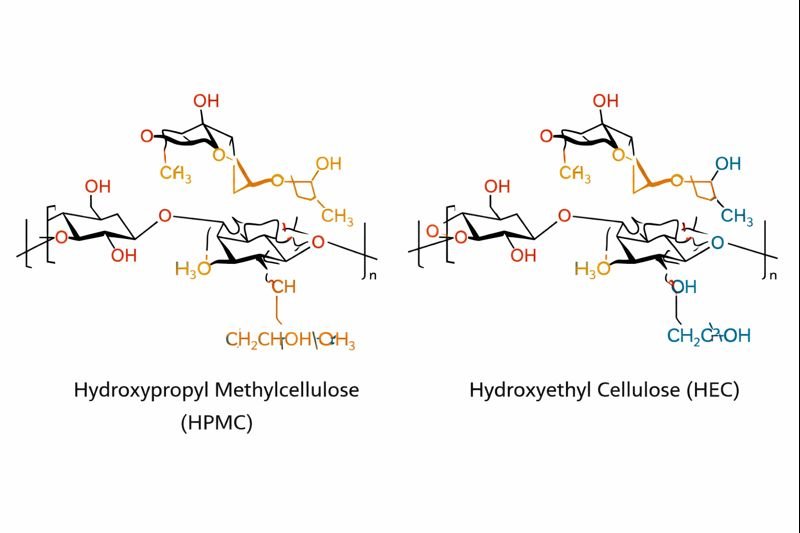
The substitution differences lead to important functional variations. In my experience working with construction material manufacturers, these differences can make or break a formulation.
HPMC forms a thermal gel at elevated temperatures (around 60-80°C), making it ideal for controlled-release applications and construction products where delayed solubility is beneficial. HEC, on the other hand, doesn't show this thermal gelation behavior.
Water retention is another critical factor - HPMC typically shows better water retention than HEC, which makes it preferred in cement-based applications. HEC, however, has superior compatibility with high-electrolyte solutions and hard water.
I remember a client who switched from HEC to HPMC in their exterior wall putty formula, then faced complaints about inconsistent workability. When we investigated, we found the water hardness variation across their market regions was affecting their product. Switching back to HEC solved the problem immediately.
Application Comparison
| Property | HPMC | HEC |
|---|---|---|
| Salt/Electrolyte Tolerance | Moderate | Excellent |
| Thermal Gelation | Yes | No |
| Water Retention | Excellent | Good |
| Enzymatic Degradation Resistance | Better | Good |
| Ideal Applications | Cement-based materials, pharmaceuticals | Paints, personal care products in varying water conditions |
Does Soap Lather Better in Hard or Soft Water?
Anyone who's traveled between regions knows that shower experiences can vary dramatically. The soap that creates rich lather at home might barely bubble in a hotel bathroom.
Soap lathers significantly better in soft water. This happens because soft water contains minimal calcium and magnesium ions that would otherwise bind with soap molecules to form insoluble soap scum. Without these interfering minerals, soap can create abundant, rich lather.

The impact of water hardness extends far beyond just a pleasant shower experience. For manufacturers, water hardness consistency is crucial for product quality and customer satisfaction.
In industrial applications, this is why many production facilities install water softening systems. I've consulted for several personal care manufacturers who saved thousands in raw material costs by softening their process water. When using soft water, they could reduce the concentration of surfactants while maintaining the same performance.
The chemical mechanism is straightforward but impactful: when soap's carboxylate groups remain free (not bound to calcium or magnesium), they can perform their intended functions of reducing surface tension and forming micelles. These micelles trap dirt and oils, allowing them to be washed away.
For consumers in hard water regions, this explains why they often need to use more product to achieve the same cleaning effect - a hidden cost of hard water that adds up over time.
Effects of Water Hardness on Cleaning Products
| Water Type | Soap Performance | Synthetic Detergent Performance | HEC-Thickened Product Performance |
|---|---|---|---|
| Soft Water | Excellent lathering, efficient cleaning | Good performance | Consistent viscosity |
| Hard Water | Poor lathering, soap scum formation | Slightly reduced efficiency | Consistent viscosity |
| Very Hard Water | Minimal lather, significant scum | Moderately reduced efficiency | Consistent viscosity |
Why Do Some Products Not Form Lather With Hard Water?
I often have customers complain that their formulations perform inconsistently across different regions. This widespread problem has a specific chemical cause.
Products fail to lather in hard water because calcium and magnesium ions neutralize the negative charges of anionic surfactants, causing them to precipitate rather than form micelles. This precipitation prevents the formation of the air-trapping structures needed for lather creation.
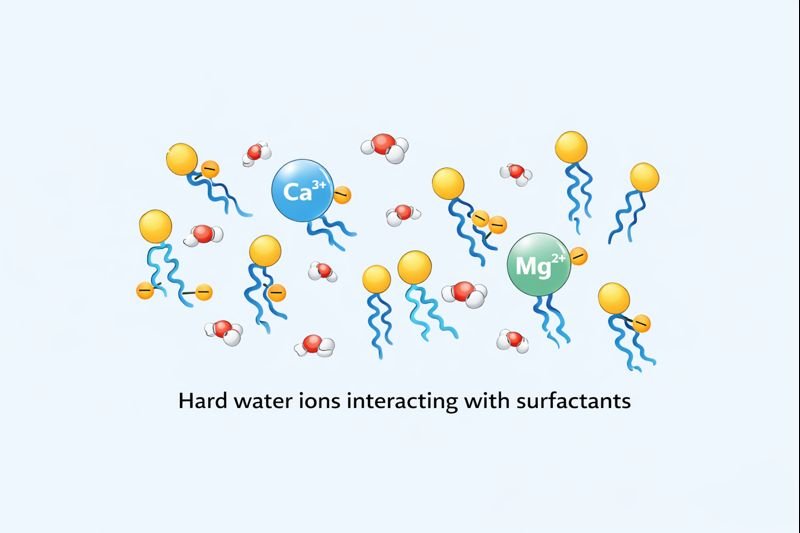
This issue extends beyond just poor lathering. The precipitates formed can leave residues on surfaces, clothes, and even hair, making them feel rough or look dull. For industrial customers, this translates directly to customer complaints and returns.
The solution lies in understanding the ionic interactions at play. Non-ionic thickeners like HEC remain stable because they have no charged groups to interact with the hard water ions. This is precisely why HEC is so valuable in formulations destined for global markets with varying water conditions.
I remember working with a client who exported personal care products to regions across the Middle East, where water hardness can be extreme. They were battling constant complaints about product performance until we reformulated using HEC as the primary thickener and added chelating agents to handle the calcium and magnesium ions. The complaints disappeared almost overnight.
For manufacturers concerned about water hardness, there are several strategies:
- Use non-ionic thickeners like HEC
- Include water softeners or chelating agents in formulations
- Increase surfactant concentrations to overcome the hard water effects
- Use surfactant blends with higher hard water tolerance
The most cost-effective approach usually involves non-ionic ingredients like HEC, which simply bypass the entire problem rather than trying to fix it after the fact.
Conclusion
HEC thickener works consistently in all water conditions because it's non-ionic—those calcium and magnesium ions in hard water that disrupt other thickeners simply can't interact with HEC's neutral structure, ensuring reliable performance everywhere.
FAQ
Can HEC be used in all types of water-based formulations?
Yes, HEC is compatible with most water-based formulations and remains stable across pH ranges from 2-12.
Does HEC affect the pH of formulations?
HEC is pH neutral and doesn't significantly impact the pH of final formulations.
What concentration of HEC is typically needed?
Most applications require 0.5-2% HEC, though exact concentrations depend on the desired viscosity.
Is HEC biodegradable?
Yes, HEC is biodegradable, making it environmentally friendly compared to some synthetic thickeners.
Can HEC be combined with other thickeners?
Yes, HEC works well in combination with other thickeners to achieve specific rheological profiles.







