Struggling with slow-setting concrete on your job site? Watching project timelines slip because mortar won't harden fast enough in cold weather? These common problems cost you money and customer satisfaction every day.
Calcium formate1 functions as a setting accelerator in concrete and mortar mixtures, reducing hardening time especially in cold weather. It improves early strength development without compromising long-term durability, making it valuable for construction projects with tight schedules.

I've been in the construction materials industry for years, and one question keeps coming up from my clients: what exactly does calcium formate do, and why is it worth paying for? Let me share what I've learned from supplying this crucial additive to major construction projects2 worldwide.
What Is the Use of Calcium Formate in Concrete?
Are you pouring concrete in winter conditions and worried about freezing before it sets? Or perhaps you're facing tight project deadlines that require faster strength development? These situations demand a reliable accelerator.
Calcium formate1 accelerates the hydration process of cement, shortening setting times by 30-50% depending on dosage. It catalyzes the reaction between cement and water, forming calcium silicate hydrate gel more rapidly, which is crucial for developing early compressive strength.
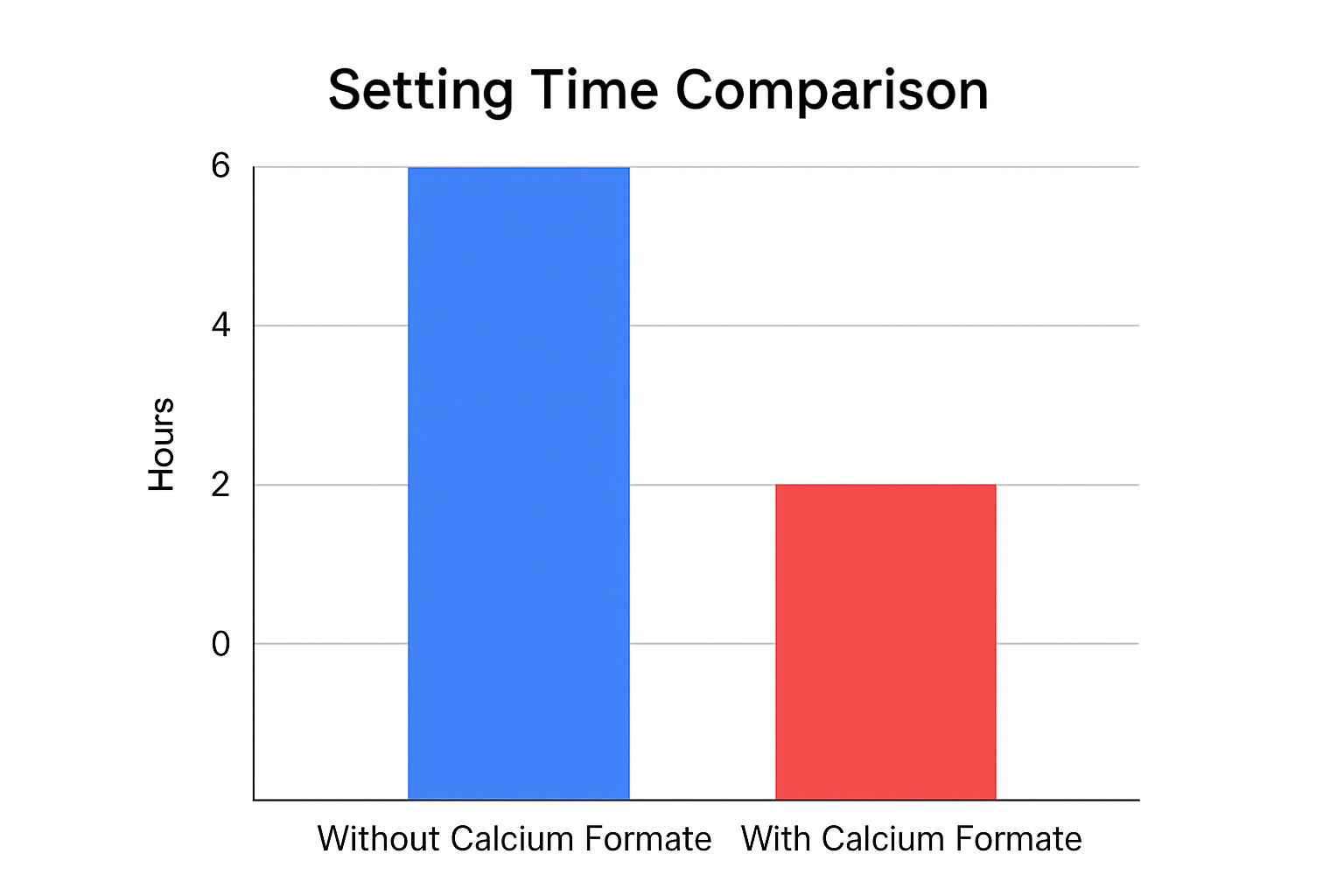
Calcium formate works differently from other accelerators like calcium chloride. While both speed up concrete setting, calcium formate doesn't introduce harmful chlorides that can corrode reinforcing steel. This makes it especially valuable for reinforced concrete structures where long-term durability is essential.
I remember visiting a major construction site in Saudi Arabia where temperatures dropped significantly at night, causing serious delays in their concrete work. After recommending our synthetic calcium formate, they saw setting times reduce by almost 40%, allowing them to maintain their construction schedule even during the coldest hours.
The chemistry behind this is fascinating. Calcium formate participates directly in cement hydration reactions, particularly with C3S (tricalcium silicate) and C3A (tricalcium aluminate) - the primary compounds responsible for strength development in cement. By accelerating these reactions, it helps concrete reach initial set faster and develop early strength more efficiently.
Optimal Dosage Recommendations for Different Applications
| Application Type | Recommended Dosage (% by cement weight) | Expected Setting Time Reduction |
|---|---|---|
| General construction | 0.5-1.0% | 20-30% |
| Cold weather concreting | 1.0-2.0% | 30-50% |
| Prefabricated elements | 1.5-2.5% | 40-60% |
Remember that overdosing can lead to flash setting and reduced workability, so proper testing before full-scale application is always recommended.
What Are the Benefits of Calcium Formate?
Have you ever had to demolish and rebuild because your concrete didn't achieve sufficient early strength before freezing? Or faced penalties for project delays that could have been avoided with faster-setting mixes? These costly problems have simple solutions.
Calcium formate provides multiple benefits: it accelerates setting without causing corrosion, improves early strength by up to 40% at 24 hours, reduces water requirements by 5-10%, enhances freeze-thaw resistance, and is environmentally safer than chloride-based alternatives.

Beyond the primary acceleration effects, calcium formate offers several secondary benefits that make it a valuable addition to concrete and mortar formulations. One significant advantage is its non-corrosive nature compared to calcium chloride, which has been the traditional go-to accelerator for decades. This makes calcium formate particularly valuable for reinforced concrete structures where steel corrosion is a major concern.
I once supplied a large batch of synthetic calcium formate to a client building a coastal structure in the UAE. They were initially considering calcium chloride but were worried about long-term durability in the salt-rich environment. After switching to calcium formate, not only did they get the acceleration they needed, but five years later, the structure shows none of the typical chloride-induced corrosion issues common in that environment.
Calcium formate also contributes to improved freeze-thaw resistance in concrete, which is crucial for structures in variable climates. It does this by accelerating the cement hydration process, allowing the concrete to develop sufficient strength before being exposed to freezing temperatures. This reduces the risk of damage during the critical early curing period.
Another benefit worth noting is that calcium formate is environmentally friendlier than many alternative accelerators. It's biodegradable and doesn't introduce harmful substances into the concrete that might later leach into soil or groundwater. This makes it increasingly popular in green building projects where environmental impact is carefully considered.
Quality Comparison: Synthetic vs. By-product Calcium Formate
| Property | Synthetic Calcium Formate | By-product Calcium Formate |
|---|---|---|
| Purity | >98% | 85-95% |
| Appearance in solution | Clear | Cloudy, yellowish |
| Setting time consistency | Highly predictable | Variable |
| Long-term efflorescence risk | Minimal | Moderate to high |
| Cost | Higher | Lower |
Why Do They Put Calcium in Concrete?
Do your clients complain about white efflorescence forming on concrete surfaces? Or have you noticed strength inconsistencies between batches despite using the same mix design? These issues often trace back to calcium compound quality and selection.
Calcium compounds like calcium formate are added to concrete primarily to control setting time and strength development. Calcium is essential in cement chemistry, forming calcium silicate hydrates that provide concrete's structural integrity while influencing workability, durability, and final appearance.

Calcium plays several fundamental roles in concrete technology. At its core, portland cement itself is primarily composed of calcium compounds - mainly calcium silicates and calcium aluminates. When water is added to cement, these compounds undergo hydration reactions, forming calcium silicate hydrate (C-S-H) gel, which is the primary binding agent responsible for concrete's strength.
Beyond the calcium naturally present in cement, additional calcium-based admixtures like calcium formate are added to modify specific properties of the concrete mix. The efficacy of these admixtures largely depends on their quality and how they interact with the cement chemistry.
I've witnessed firsthand how using low-quality, by-product calcium formate can lead to serious problems. Last year, one of my clients switched suppliers to save costs, purchasing by-product calcium formate instead of synthetic. Within weeks, they faced complaints about white efflorescence forming on walls. Laboratory analysis confirmed that impurities in the by-product calcium formate were migrating to the surface as the concrete dried.
The quality difference between synthetic and by-product calcium formate is significant. Synthetic calcium formate is produced through a controlled chemical reaction between calcium hydroxide and formic acid, resulting in a high-purity product typically exceeding 98%. By-product calcium formate, in contrast, is recovered from various industrial processes and often contains impurities that can affect concrete performance and appearance.
Testing Calcium Formate Quality
A simple test to distinguish quality is dissolving a sample in water:
- High-quality synthetic calcium formate produces a clear solution
- By-product calcium formate typically creates a cloudy, yellowish solution due to impurities
Always specify "synthetic calcium formate" in your purchasing contracts to avoid these issues. The slightly higher cost is insignificant compared to the potential remediation expenses and reputation damage from failed projects.
What Is the Purpose of Calcium Hydroxide in Concrete?
Have your structures ever suffered from premature deterioration in acidic environments? Or wondered why some concrete maintains its strength for decades while others gradually weaken? The answer often lies in calcium hydroxide management.
Calcium hydroxide forms naturally during cement hydration, comprising 15-25% of hydrated cement paste. It maintains the high alkalinity (pH 12-13) necessary to protect reinforcing steel from corrosion while serving as a reservoir for continued strength development through pozzolanic reactions.
Calcium hydroxide, also known as portlandite, is a crucial component in the complex chemistry of concrete. When cement hydrates, it produces calcium silicate hydrate (C-S-H) gel and calcium hydroxide. While C-S-H provides most of concrete's strength, calcium hydroxide plays several critical supporting roles.
The most important function of calcium hydroxide is maintaining concrete's alkalinity. This high pH environment creates a passive oxide layer on embedded steel reinforcement, effectively protecting it from corrosion. When concrete carbonates (reacts with atmospheric CO2), calcium hydroxide is consumed, reducing alkalinity and potentially compromising this protection.
I recently consulted on a 40-year-old structure in Pakistan that showed minimal corrosion despite harsh environmental conditions. Analysis revealed that the concrete contained supplementary cementitious materials that had reacted with calcium hydroxide over decades, creating additional C-S-H gel and densifying the concrete matrix. This pozzolanic reaction, where materials like fly ash, silica fume, or metakaolin combine with calcium hydroxide, is a key mechanism for long-term strength development and durability improvement.
However, calcium hydroxide can also contribute to some concrete vulnerabilities. Being more soluble than other cement hydration products, it can leach out in acidic environments, leaving voids that weaken the concrete structure. It's also involved in various durability issues, including alkali-silica reaction when reactive aggregates are present.
The relationship between calcium formate and calcium hydroxide is interesting - calcium formate accelerates cement hydration, which initially produces more calcium hydroxide. However, when properly formulated with appropriate supplementary cementitious materials, this additional calcium hydroxide can participate in pozzolanic reactions, ultimately creating more durable concrete.
Balancing Calcium Hydroxide in Concrete Formulations
| Consideration | Too Little Calcium Hydroxide | Optimal Range | Too Much Calcium Hydroxide |
|---|---|---|---|
| Steel protection | Corrosion risk increases | Passive protection maintained | Potential for excess leaching |
| Long-term strength | Limited pozzolanic potential | Sufficient for ongoing reactions | May lead to efflorescence |
| Acid resistance | Better initially, worse long-term | Balanced performance | Poor resistance |
| Carbonation resistance | Reduced | Adequate | Excessive carbonation shrinkage |
Conclusion
Calcium formate1 is essential in concrete as a non-corrosive accelerator that improves early strength and workability. Always specify synthetic calcium formate to avoid quality issues and ensure consistent, durable concrete performance.







